Consider AMPYRA® (dalfampridine) for all your appropriate patients with MS-related walking difficulty
In clinical trials, improvements in walking speed were demonstrated in patients:3-5
- With all 4 major types of MS
- With ages ranging from 25 to 73 years
- With or without use of disease modifying therapies (DMTs)*
- With EDSS scores ranging from 2.5 to 6.5
*DMTs included in the clinical trials: interferons, glatiramer acetate, or natalizumab.
Selected Important Safety Information
- Clinical studies of AMPYRA did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Because elderly patients are more likely to have decreased renal function, it is important to know the estimated CrCl before initiating AMPYRA.
Please see additional Important Safety Information below.
AMPYRA® (dalfampridine) improved walking speed in significantly more patients than placebo in 2 clinical studies.3-5
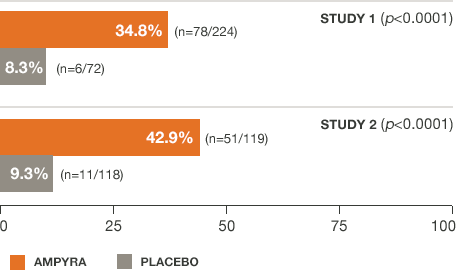
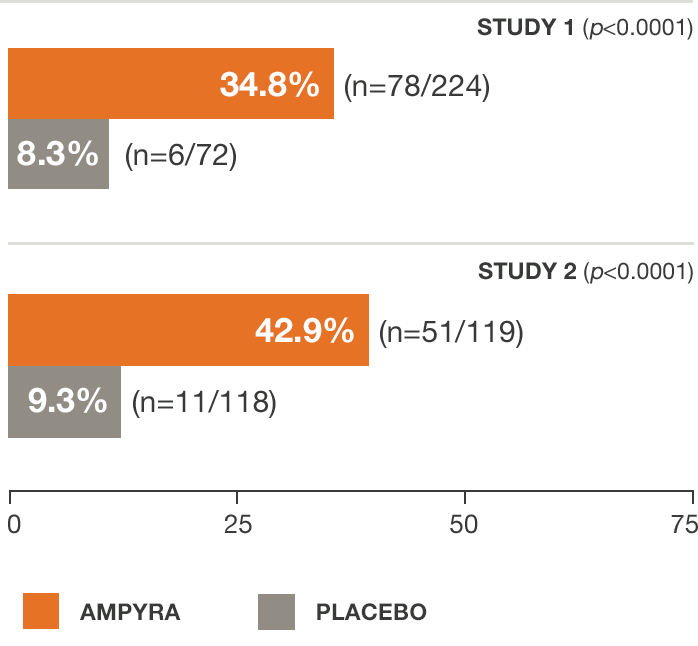
Not every patient responds to AMPYRA.
Individual patient response to the therapy may vary.
25%
Average increase in walking speed3-5
Patients responding to AMPYRA increased walking speed by an average of approximately 25% from baseline in two clinical studies.3-5
- A responder was defined as a patient whose walking speed on the Timed 25-Foot Walk (T25FW) was faster for at least 3 of 4 visits during treatment than the fastest speed measured during 5 off-drug visits.
Description of studies
AMPYRA® (dalfampridine) was evaluated in 2 randomized, placebo-controlled studies including a total of 540 patients with disease durations ranging from 0.1-45.6 years (mean of 13) and Kurtzke Expanded Disability Status Scale (EDSS) scores ranging from 1.5-7.0 (mean of 6).
Inclusion required a baseline Timed 25-Foot Walk (T25FW) between 8 and 45 seconds. Exclusion criteria included a history of seizures, EEG evidence of epileptiform activity, or an MS exacerbation within the past 60 days. Study 2 also excluded patients with severe renal impairment.
Study 1 was a 21-week study with 14 weeks of double-blind treatment with AMPYRA 10 mg twice daily (n=229) or placebo (n=72). A total of 283 patients completed all study visits (212 AMPYRA and 71 placebo). Study 2 was a 14-week study with 9 weeks of double-blind treatment with AMPYRA 10 mg twice daily (n=120) or placebo (n=119). A total of 227 patients completed all study visits (113 AMPYRA and 114 placebo).
The primary measure of efficacy in both studies was walking speed (in feet per second) as measured by the T25FW, using a responder analysis. A responder was defined as a patient whose walking speed on the T25FW was faster for at least 3 of 4 visits during treatment than the fastest speed measured during 5 off-drug visits.3-5
Discover what improved walking speed could mean to patients
Regardless of baseline disability, AMPYRA® (dalfampridine) responders increased walking speed by an average of about 25%6
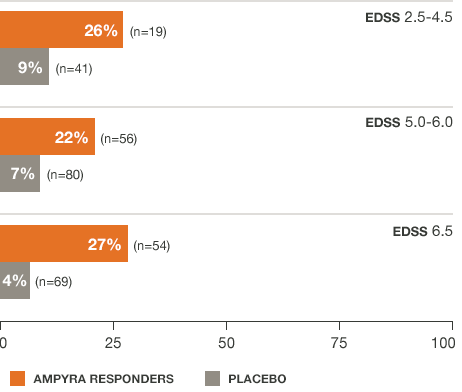
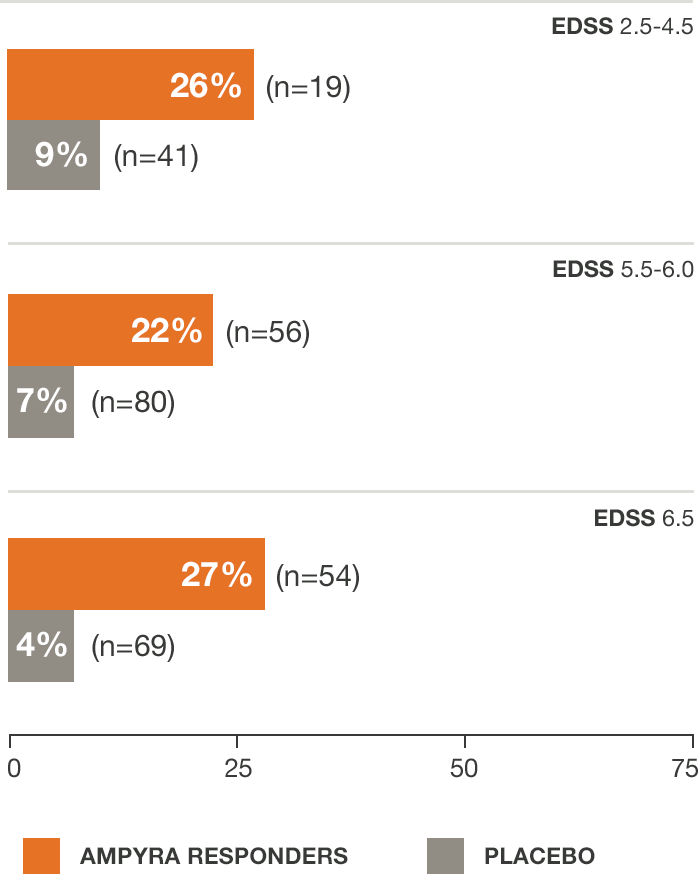
*Pooled data from studies 1 and 2.
25%
Average increase in walking speed3-5
Patients responding to AMPYRA increased walking speed by an average of approximately 25% from baseline in two clinical studies.3-5
- A responder was defined as a patient whose walking speed on the Timed 25-Foot Walk (T25FW) was faster for at least 3 of 4 visits during treatment than the fastest speed measured during 5 off-drug visits.
Individual patient response to therapy may vary.
For AMPYRA non-responders, average % change from baseline walking speed was 11.7, 8.2, and 3.2 for EDSS scores of 2.5-4.5 (n=37), 5.0-6.0 (n=99), and 6.5 (n=78), respectively.
Description of studies
AMPYRA® (dalfampridine) was evaluated in 2 randomized, placebo-controlled studies including a total of 540 patients with disease durations ranging from 0.1-45.6 years (mean of 13) and Kurtzke Expanded Disability Status Scale (EDSS) scores ranging from 1.5-7.0 (mean of 6).
Inclusion required a baseline Timed 25-Foot Walk (T25FW) between 8 and 45 seconds. Exclusion criteria included a history of seizures, EEG evidence of epileptiform activity, or an MS exacerbation within the past 60 days. Study 2 also excluded patients with severe renal impairment.
Study 1 was a 21-week study with 14 weeks of double-blind treatment with AMPYRA 10 mg twice daily (n=229) or placebo (n=72). A total of 283 patients completed all study visits (212 AMPYRA and 71 placebo). Study 2 was a 14-week study with 9 weeks of double-blind treatment with AMPYRA 10 mg twice daily (n=120) or placebo (n=119). A total of 227 patients completed all study visits (113 AMPYRA and 114 placebo).
The primary measure of efficacy in both studies was walking speed (in feet per second) as measured by the T25FW, using a responder analysis. A responder was defined as a patient whose walking speed on the T25FW was faster for at least 3 of 4 visits during treatment than the fastest speed measured during 5 off-drug visits.3-5
Discover what improved walking speed could mean to patients
Regardless of DMT use,* AMPYRA® (dalfampridine) responders increased walking speed by an average of about 25%7
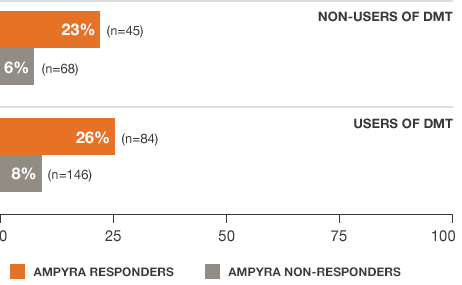
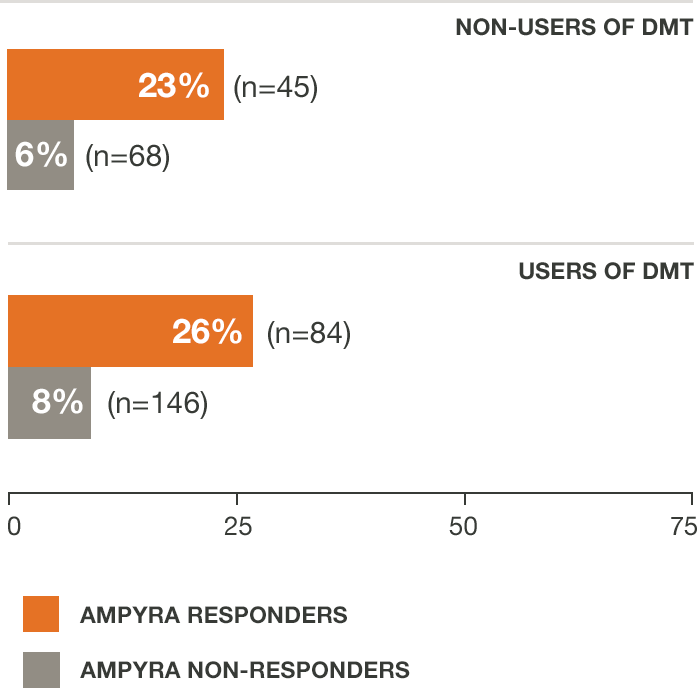
*AMPYRA was effective in improving walking when used with DMTs interferons, glatiramer acetate, naralizumab, or when used alone.
†Pooled data from studies 1 and 2.
25%
Average increase in walking speed3-5
Patients responding to AMPYRA increased walking speed by an average of approximately 25% from baseline in two clinical studies.3-5
- A responder was defined as a patient whose walking speed on the Timed 25-Foot Walk (T25FW) was faster for at least 3 of 4 visits during treatment than the fastest speed measured during 5 off-drug visits.
Description of studies
AMPYRA® (dalfampridine) was evaluated in 2 randomized, placebo-controlled studies including a total of 540 patients with disease durations ranging from 0.1-45.6 years (mean of 13) and Kurtzke Expanded Disability Status Scale (EDSS) scores ranging from 1.5-7.0 (mean of 6).
Inclusion required a baseline Timed 25-Foot Walk (T25FW) between 8 and 45 seconds. Exclusion criteria included a history of seizures, EEG evidence of epileptiform activity, or an MS exacerbation within the past 60 days. Study 2 also excluded patients with severe renal impairment.
Study 1 was a 21-week study with 14 weeks of double-blind treatment with AMPYRA 10 mg twice daily (n=229) or placebo (n=72). A total of 283 patients completed all study visits (212 AMPYRA and 71 placebo). Study 2 was a 14-week study with 9 weeks of double-blind treatment with AMPYRA 10 mg twice daily (n=120) or placebo (n=119). A total of 227 patients completed all study visits (113 AMPYRA and 114 placebo).
The primary measure of efficacy in both studies was walking speed (in feet per second) as measured by the T25FW, using a responder analysis. A responder was defined as a patient whose walking speed on the T25FW was faster for at least 3 of 4 visits during treatment than the fastest speed measured during 5 off-drug visits.3-5
Discover what improved walking speed could mean to patients
Description of studies
AMPYRA® (dalfampridine) was evaluated in 2 randomized, placebo-controlled studies including a total of 540 patients with disease durations ranging from 0.1-45.6 years (mean of 13) and Kurtzke Expanded Disability Status Scale (EDSS) scores ranging from 1.5-7.0 (mean of 6).
Inclusion required a baseline Timed 25-Foot Walk (T25FW) between 8 and 45 seconds. Exclusion criteria included a history of seizures, EEG evidence of epileptiform activity, or an MS exacerbation within the past 60 days. Study 2 also excluded patients with severe renal impairment.
Study 1 was a 21-week study with 14 weeks of double-blind treatment with AMPYRA 10 mg twice daily (n=229) or placebo (n=72). A total of 283 patients completed all study visits (212 AMPYRA and 71 placebo). Study 2 was a 14-week study with 9 weeks of double-blind treatment with AMPYRA 10 mg twice daily (n=120) or placebo (n=119). A total of 227 patients completed all study visits (113 AMPYRA and 114 placebo).
The primary measure of efficacy in both studies was walking speed (in feet per second) as measured by the T25FW, using a responder analysis. A responder was defined as a patient whose walking speed on the T25FW was faster for at least 3 of 4 visits during treatment than the fastest speed measured during 5 off-drug visits.3-5
Indication
AMPYRA® (dalfampridine) Extended Release Tablets, 10 mg, is indicated to improve walking in adults with multiple sclerosis (MS). This was demonstrated by an increase in walking speed.
Important Safety Information
AMPYRA is contraindicated in patients with history of seizure, moderate or severe renal impairment (CrCl ≤ 50 mL/min), or history of hypersensitivity to AMPYRA or 4-aminopyridine.
Indication
AMPYRA® (dalfampridine) Extended Release Tablets, 10 mg, is indicated to improve walking in adults with multiple sclerosis (MS). This was demonstrated by an increase in walking speed.
Important Safety Information
- AMPYRA is contraindicated in patients with history of seizure, moderate or severe renal impairment (CrCl ≤ 50 mL/min), or history of hypersensitivity to AMPYRA or 4-aminopyridine.
- AMPYRA can cause seizures. The risk of seizures increases with increasing doses. Permanently discontinue AMPYRA if seizure occurs. In the post-marketing period seizures have been reported. The majority of seizures occurred at the recommended dose, in patients without a history of seizures, and generally within days to weeks of starting therapy.
- AMPYRA has not been evaluated in patients with history of seizures or with epileptiform activity on an EEG, as these patients were excluded from clinical trials. The risk of seizures in patients with epileptiform activity on an EEG is unknown, and could be substantially higher than that observed in clinical studies.
- Avoid concomitant use of AMPYRA with other forms of 4-aminopyridine (4-AP, fampridine), since the active ingredient is the same. Instruct patients to discontinue use of any product containing 4-AP prior to initiating AMPYRA to reduce the potential for dose-related adverse reactions.
- AMPYRA can cause anaphylaxis and severe allergic reaction. Signs and symptoms included respiratory compromise, urticaria, and angioedema of the throat or tongue. If an anaphylactic or other serious allergic reaction occurs, permanently discontinue AMPYRA.
- AMPYRA is cleared predominantly by the kidneys. The risk of seizures in patients with mild renal impairment (CrCl 51–80 mL/min) is unknown, but AMPYRA plasma levels in these patients may approach those seen at a dose of 15 mg twice daily, a dose that may be associated with an increased risk of seizures. Estimated CrCl should be known before initiating AMPYRA and monitored at least annually during treatment.
- The most common adverse reactions (incidence ≥ 2% and at a rate greater than placebo) for AMPYRA in MS patients were urinary tract infection, insomnia, dizziness, headache, nausea, asthenia, back pain, balance disorder, MS relapse, paresthesia, nasopharyngitis, constipation, dyspepsia, and pharyngolaryngeal pain.
- The risk of adverse reactions, including seizures, increases with increasing AMPYRA doses. There is no evidence of additional benefit at doses greater than 10 mg twice daily.
- Concomitant use with OCT2 inhibitors (e.g., cimetidine) may cause increased exposure to AMPYRA and potential risk of seizures.
- There are no adequate data on AMPYRA in pregnant women. Based on animal data, use of AMPYRA during pregnancy may cause fetal harm.
- There are no data on presence of AMPYRA in breastmilk; benefits of breastfeeding should be considered along with benefit of AMPYRA to the mother and potential risks to the infant.
- Safety and effectiveness of AMPYRA in patients younger than 18 years have not been established.
- Clinical studies of AMPYRA did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Because elderly patients are more likely to have decreased renal function, it is important to know the estimated CrCl before initiating AMPYRA.
Please see the Full Prescribing Information.
